 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeIsomerisation of alkanes involves the conversion of straight-chain alkanes into isomeric branched chain alkanes.
For example, when butane is heated with aluminium chloride in the presence of dry HCl gas at 573K under about 35 atmospheres pressure, 2-methyl propane is produced.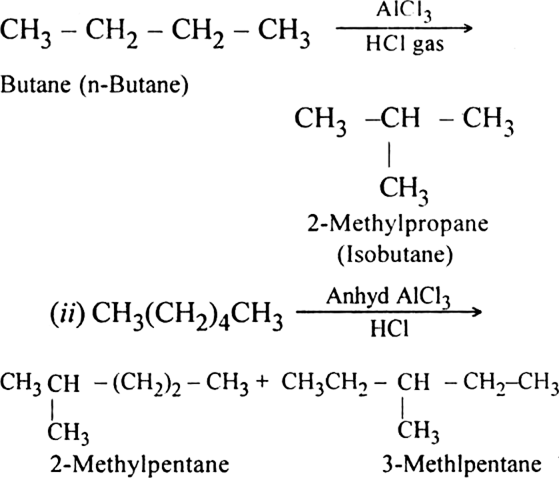
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeOut of staggered and eclipsed conformations of ethane, which is more stable and why ?
 Short Answer Type
Short Answer Type