 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow will you explain that there exists two varieties of 1, 2,-dichloroethene while there is only one type of 1, 2-dichloroethane?
The structure of 1, 2-dichloroethene is![]()
Since in the above structure there is C = C bond and two chlorine atoms are present at two different carbon atoms, therefore, it is capable of exhibiting geometrical isomerism. The two geometrical isomers (i.e. two varieties) of the above compound can be represented as: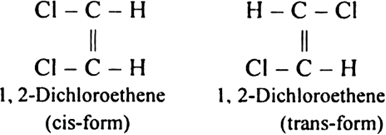
The structure of 1, 2-dichloroethane is![]()
The molecule has C-C bond. As the rotation of the atoms or groups about single bond is quite free, the compound fails to show geometrical isomerism.
 Short Answer Type
Short Answer TypeDraw cis and trans isomers of the following compounds. Also, write their IUPAC names:
(i) CHCl = CHCl
(ii) C2H5CCH3 = CCH3C2H5
Which of the following compounds will show cis-trans isomerism?
(i)(CH3)2C = CH-C2H5
(ii) CH2 = CBr2
(iii) C2H5CH = CH-CH2
(iv) CH3CH = CClCH3
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
What happens when 2-Bromobutane is heated with alcoholic KOH. Account for the product formed.
What happens when butan-2-ol is heated with concentrated H2SO4. Account for the product formed.
