 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow will you explain that there exists two varieties of 1, 2,-dichloroethene while there is only one type of 1, 2-dichloroethane?
 Short Answer Type
Short Answer TypeThe compounds (i) 2-Butene and (iv) 3, 4-Dimethyl-3-hexene would show geometrical isomerism. In both the cases, the two atoms or groups attached to the double bonded carbon atoms are different.
(i) But -2- ene (CH3 - CH = CH - CH3) exhibits geometrical isomerism.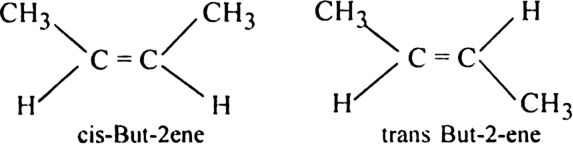
(iv) 3, 4-Dimethylhex-3-ene: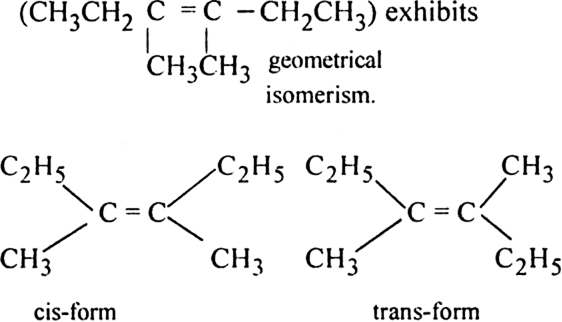
Draw cis and trans isomers of the following compounds. Also, write their IUPAC names:
(i) CHCl = CHCl
(ii) C2H5CCH3 = CCH3C2H5
Which of the following compounds will show cis-trans isomerism?
(i)(CH3)2C = CH-C2H5
(ii) CH2 = CBr2
(iii) C2H5CH = CH-CH2
(iv) CH3CH = CClCH3
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
What happens when 2-Bromobutane is heated with alcoholic KOH. Account for the product formed.
What happens when butan-2-ol is heated with concentrated H2SO4. Account for the product formed.
