 Long Answer Type
Long Answer TypeDiscuss the free radical mechanism of addition of HBr to propene.
Or
Give the mechanism of addition of HBr to propylene in the presence of peroxide.
Propylene reacts with HBr in the presence of peroxide to form 1-bromo propane.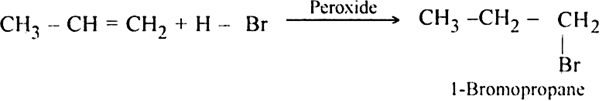
Mechanism: It proceeds with free radical mechanism and consists of the following steps:
Step I. Chain initiating step: Organic peroxide undergoes homolysis to from free radicals.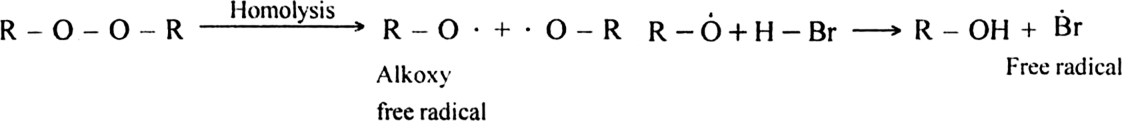
Step II. Chain propagating step: The bromine radical then adds to propene molecule in such a way that the more stable free radical is produced. 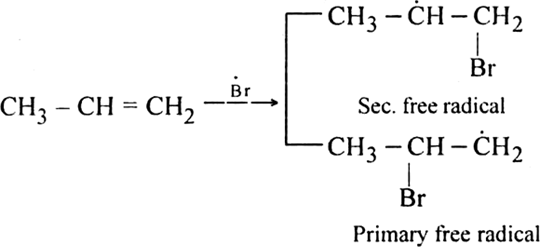
As the secondary free radical is more stable, it will be preferably formed. The secondary free radical then reacts with HBr to form 1-bromo-propane and bromine radical.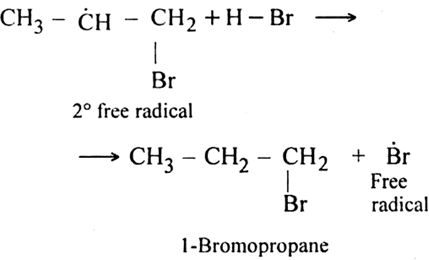
This bromine radical continues the chain by repeating the chain propagating step.
Step III. Chain terminating step: At the end, the reaction stops by coupling the radicals.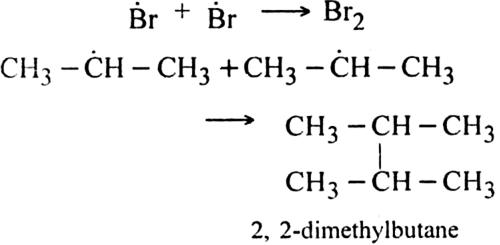
The addition of HBr to propene yields 2-broniopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
 Short Answer Type
Short Answer TypeWhy peroxide effect is shown only by HBr and not by HCl or HI?
Or
Explain why Kharasch effect is shown by HBr only and not by HCl or HI.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWrite IUPAC names of the products obtained by the ozonolysis of the following compounds:
Pent - 2-ene
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
3, 4-Dimethlhept-3-ene
