 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss in brief the polymerisation reactions of alkenes ?
Polymerisation: Polymerization is the process in which a large number of same or different molecules of unsaturated compounds combine to form a bigger molecule called polymer. The combining units are called monomers.
(i) Formation of polyethene: Ethylene polymerises under high pressure in the presence of traces of oxygen as a catalyst to give polythene or polyethylene.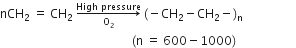
Polythene is mainly employed for the manufacture of toys, bottles, plastic pipes, polythene bags, etc.
(ii) Formation of Teflon. It is obtained by the polymerisation of tetrafluoroethylene.
It is highly resistant to heat and electricity and is used for insulating electric wires. Due to its chemical inertness, it is used for coating the inner surfaces of the non-sticking cooking pans.
(iii) Formation of polyvinyl chloride (PVC). When vinyl chloride is heated in an inert solvent in the presence of benzoyl peroxide catalyst, polyvinylchloride is formed.
It is used in making rain-coats, hand bags, table clothes, plastic dolls etc.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeFor the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type