 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are alkynes?
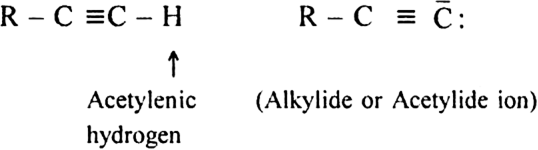
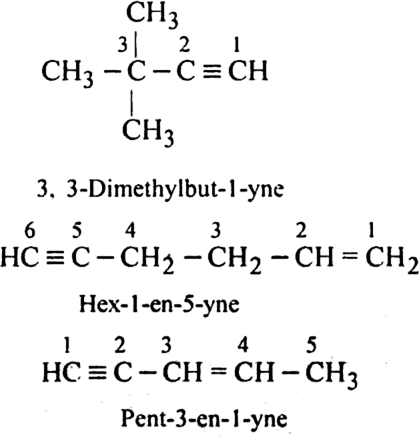
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type