 Long Answer Type
Long Answer TypeWhat are the characteristics of a compound to be aromatic?
Or
What do you mean by aromaticity?
Or
What are the necessary conditions for any system to be aromatic?
The compounds possessing aromatic character show the following characteristics:
(i)Â The compounds must be cyclic in nature and have flat planar structure.
(ii)Â Their molecular formulae suggest these compounds are highly unsaturated due to the presence of one or more double bonds in the ring but they must behave as saturated compounds.
(iii)Â They must resist addition reaction and take part in the electrophilic substitution reactions.
(iv) The molecules have delocalised  electron cloud above and below the plane of the ring.
electron cloud above and below the plane of the ring.
(v) An essential criterion for the aromatic character is that the compound must obey Huckel’s rule. According to this rule, a cyclic compound will behave as aromatic compound if it contains (4n + 2) electrons, where n may be 0, 1, 2, 3 etc. Huckel’s rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
electrons, where n may be 0, 1, 2, 3 etc. Huckel’s rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
(a) Monocyclic systems: Some monocyclic systems having π-electrons (obey Huckel’s rule) possess aromatic character.
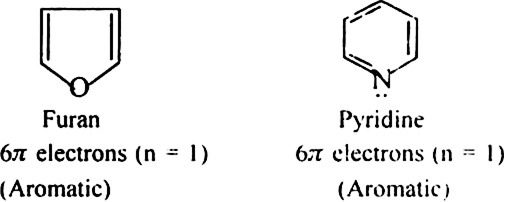
(b) Fused ring systems: The polynuclear hydrocarbons such as naphthalene, anthracene and phenanthrene are also aromatic according to Huckel’s rule.
Aromatic ions: Some cyclic ions also exhibit aromatic character. For example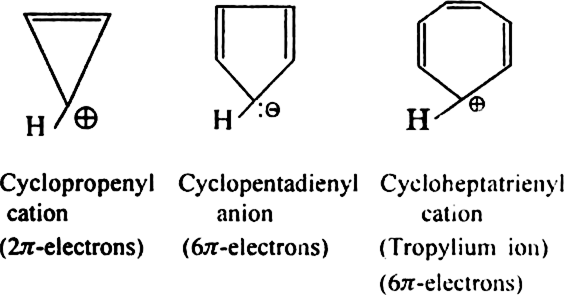
The following compounds are not aromatic: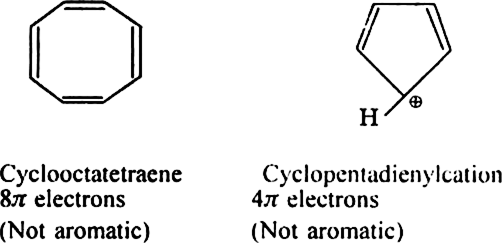
Cycloheptatriene although obeys Huckel’s rule yet it is not aromatic as it is not planar and can not show resonance.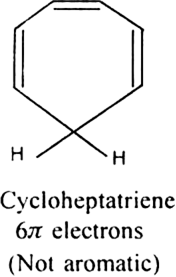
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow would you convert the following compounds to benzene:
(i) Benzoic acid
(ii) Benzene diazonium chloride?
