Short Answer Type
Short Answer TypeWhat happens when:
Benzene is treated with n-propyl chloride in the presence of anhydrous AlCl3.
 Long Answer Type
Long Answer TypeHow benzene is converted into benzene sulphonic acid? Give its mechanism.
Or
Explain the mechanism of sulphonation of benzene.
Discuss the mechanism of Friedel Craft’s reaction.
Or
Discuss the mechanism of Friedel Craft’s alkylation of benzene. Give the mechanism of reaction of benzene within ![]() the presence of AlCl3.
the presence of AlCl3.
Or
How is benzene converted into aceto-phenone? Name the reaction and discuss the mechanism explaining each step.
Or
Give the mechanism of acylation of benzene.
 Short Answer Type
Short Answer TypeWhy does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
 Long Answer Type
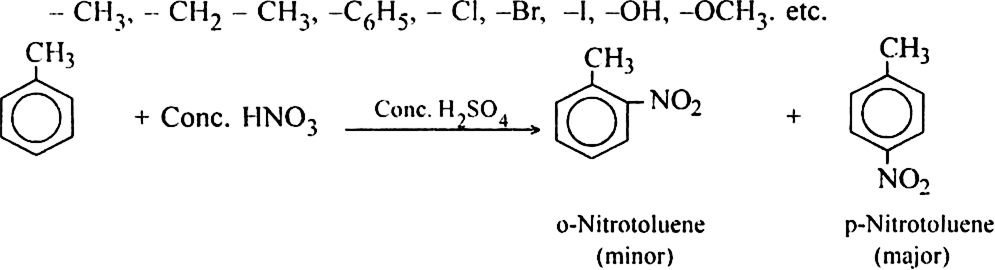
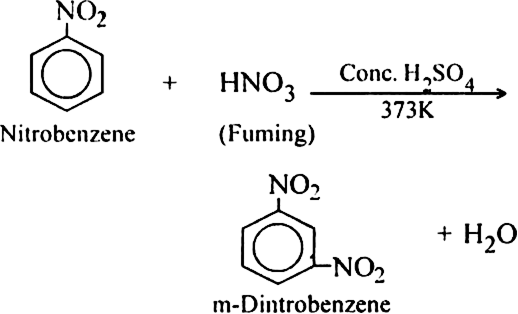
Long Answer TypeDiscuss the directive influence of substituents on disubstitution in benzene.