 Multiple Choice Questions
Multiple Choice QuestionsTwo organic compounds X and Y on analysis gave the same percentage composition namely; C = (12/13) × 100% and H = (1/13) × 100%. However, compound X decolourises bromine water while compound Y does not. The two compounds X and Y may be respectively
acetylene and ethylene
acetylene and benzene
ethylene and benzene
toluene and benzene
For preparing an alkane, a saturated solution of sodium or potassium salt of a carboxylic acid is subjected to
hydrolysis
electrolysis
oxidation
hydrogenation
An organic compound with molecular formula C6H12 upon ozonolysis give only acetone as the product. The compound is
2,3-dimethyl-1-butene
3-hexane
2-hexene
2,3-dimethyl-2-butene
An aromatic hydrocarbon with empirical formula C5H4 on treatment with concentrated H2SO4 gave a monosulphonic acid. 0.104 g of the acid required 10 mL of NaOH for complete neutralisation. The molecular formula of hydrocarbon is
C5H4
C10H8
C15H12
C20H16
C.
C15H12
Empirical formula (C5H4) + H2SO4 → Monosulphonic acid
0.104 gm of acid required 10 mL of NaOH for completely neutralisation
× 10 × 10-3
n = ≈ 3
The molecular formula of hydrocarbon will be C15H12.
An alkene having the molecular formula C9H18 on ozonolysis gives 2, 2-dimethyl propanal and 2-butanone. The alkene is :
2, 2, 2-trimethyl-3-hexene
2, 2, 6-trimethyl-3-hexane
2, 3, 4-trimethyl-2-hexene
2, 2, 4-trimethyl-3-hexene
Observe the following reactions and predict the nature of A and B:
A and B both are 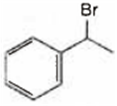
A and B both are ![]()
A is 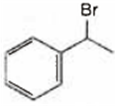 and B is
and B is ![]()
A is ![]() and B is
and B is 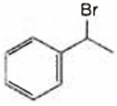
Nitration of aniline in strongly acidic medium, result in the formation of m-nitroaniline also. This is because :
amino group is meta orienting during electrophilic substitution reaction
nitro group goes always to the meta position irrespective of the substituents
nitration of aniline is a nucleophilic substitution reaction in strongly acidic medium
in strongly acidic conditions aniline is present as anilinium ion
