 Multiple Choice Questions
Multiple Choice QuestionsConsider the following reactions:
Y can be conserted to X on heating with ....... at ...... temperature :
Al2O3, 350°C
Cu, 300°C
Ca(OH)2+ CaOCl2, 60°C
NaOH/I2, 60°C
When acetylene is passed through red hot iron tube, compound X is formed. Which one of the following reactions will yield X as the major product?
2, 3-dimethyl hexane contains...... tertiary ....... secondary and ...... primary carbon atoms, respectively:
2,2,4
2,4,3
4,3,2
3,2,4
4 g of a hydrocarbon on complete combustion gave 12.571 g of CO2 and 5.143 g of water. What is the empirical formula of the hydrocarbon ?
CH
CH2
CH3
C2H3
The chemicals and the reaction conditions required for the preparation of ethane are
C2H5I, Zn-Cu, C2H5OH
CH3Cl, Na, H2O
KOOC-CH=CH-COOK, , electrolysis
CH3CO2Na, NaOH, CaO, Δ
What is the molecular formula of the product formed when benzene is reacted with ethyl chloride in presence of anhydrous aluminium chloride?
C8H10
C6H6
C8H8
C6H5Cl
The metal used for the de-brommation reaction of 1, 2-dibromoethane
Na
Zn
Mg
Li
B.
Zn
Zinc is used for de-bromination of di-bromoalkane to give alkene.
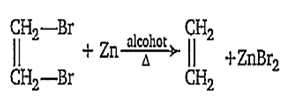
Which of the following reagents when heated with ethyl chloride, forms ethylene ?
Aqueous KOH
Zn/ HCl
Alcoholic KOH
HI
Which of the following compounds is soluble in benzene but almost insoluble in water?
C2H5OH
CH3CO2H
CH3CHO
C6H5NO2
