 Multiple Choice Questions
Multiple Choice QuestionsIn the following reaction, number of possible structure is
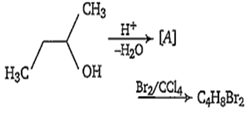
1
2
5
6
C.
5
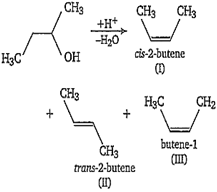
Therefore in X, order of stability of alkene is II > I > III.
All of these alkenes, with Br2/ CCl4, produce additive product having molecular formula C4H8Br2. The possible products are-
(i) CH3-CH(Br)-CH(Br)-CH3
(ii) CH3-CH(Br)-CH(Br)-CH3
(iii) BrH2C-CH(Br)-CH2-CH3
(iv) BrH2C-CH2-CHBr-CH3
(v) CH2Br-CH2-CH2-CH2Br
The IUPAC name of
 is
is
4-bromo-2-chloro-5-iodo-1-fluorobenzene
2-carbamoylhex-3-enal
1-bromo-2-chloro-3-fluoro-6-iodobenzene
2-bromo-1-chloro-5-fluoro-3-iodobenzene
Assertion (A): Reaction of 1-butene with HBr gives 1-bromobutane as major product.
Reason (R): Addition of hydrogen halides to alkenes proceeds according to Markownikoffs rule.
The correct answer is
A and R are correct and R is the correct explanation of A
A and R are correct but R is not the correct explanation of A.
A is correct but R is not correct
A is not correct but R is correct
