 Multiple Choice Questions
Multiple Choice QuestionsIn the eclipsed conformation of ethane, the dihedral angle between the hydrogen atoms of adjacent methyl groups is :
60°
120°
0°
180°
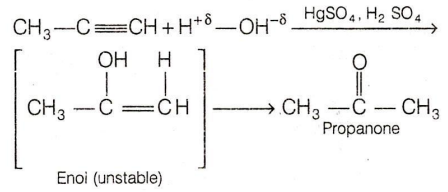
The major product of the addition of water molecule to propyne in the presence of mercuric sulphate and dilute sulphuric acid is :
ethanal
2-propanol
propane
propanone
D.
propanone
In the presence of H2SO4 and HgSO4 as a catalyst, H2O adds to triple bond of propyne to form an unstable enol which readily tautomerises to the propanone. Addition occurs according to Markownikoff 's rule.
Which of the following statements is not an essential feature of an optically active molecule ?
It will rotate the plane of polarised light
It will have a non-superimposable mirror image
It will have no element of symmetry
It will have an asymmetric carbon atom
The reagent which could distinguish between 1-hexyne and 1-hexene is :
Ag
KMnO4
Br2 in CCl4
H2SO4
Choose the correct reagent required to carry out the transformation :

Zn + conc. HCl
conc. H2SO4
Li then H2O
H2/Pt
The heat liberated when 1.89 g of benzoic acid is burnt in a bomb calorimeter at 25°C and it increases the temperature of 18.94 kg of water by 0.632°C. If the specific heat of water at 25°C is 0.998 cal/g-deg, the value of the heat of combustion of benzoic acid is
881.1 kcal
771.2 kcal
981.1 kcal
871.2 kcal
Which of the following alkenes will give same product by any method out of hydration, hydroboration-oxidation and oxymercuration-demercuration?
CH3CH=CH2
CH3CH=CHCH3
CH3CH(CH3)CH=CH2
![]()
The compound formed by the ozonolysis of acetylene :
glycol
acetic acid
ethylene ozonide
glyoxal
