 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite a short note on allotropy of hydrogen.
Or
How many allotropes of dihydrogen are known? What is their importance?
How would you prepare dihydrogen from water using a reducing agent?
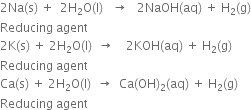
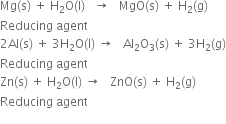
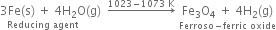

 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type