 Short Answer Type
Short Answer TypeGive two chemical reactions which show the acidic character of hydrogen peroxide.
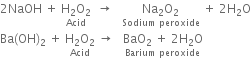

 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow is strength of hydrogen peroxide expressed? Calculate the strength of 20 volume hydrogen peroxide in grams per litre.
