 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe structure of hydrogen peroxide has been established by X-ray studies.
(i) The molecule of hydrogen peroxide is not planar. It is described as an ‘open book structure.
(ii) The O – O part of the molecule can be thought of as lying on the spine of a book open at an angle of 90°.
(iii) The hydrogen atoms are placed one on each cover.
(iv) The H – O bonds make an angle of about 101.5° with the O – O bond as shown.
(v) The O – O linkage present between two oxygen atoms is called peroxybond (peroxide linkage).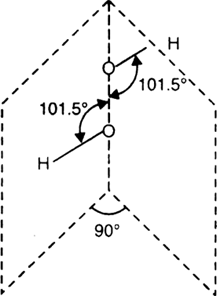
(vi) This O – O linkage results from a p-p overlap of the p-orbitals of the two oxygen atoms.
(vii) Each H – O bond results from an s-p overlap between s-orbital of hydrogen and p-orbital of the oxygen atom.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow is strength of hydrogen peroxide expressed? Calculate the strength of 20 volume hydrogen peroxide in grams per litre.
