 Multiple Choice Questions
Multiple Choice QuestionsSulphur trioxide is dissolved in heavy water to form a compound X. The hybridisation state of sulphur in X is :
sp2
sp3
sp
dsp2
Which one of the following reactions does not form gaseous product?
PbO2 + H2O2 →
Acidified KMnO4 + H2O2 →
PbS + H2O2 →
Cl2 + H2O2 →
Assertion (A) : NaCl is less soluble in heavy water than in ordinary water.
Reason (R) : Dielectric constant of ordinary water is more than that of heavy water.
The correct answer is:
Both (A) and (R) are true and (R) is the correct explanation of (A)
Both (A) and (R) are true but (R) is not the correct explanation of (A)
(A) is true, but (R) is not true
(A) is not true, but (R) is true
Which one of the following reactions represents the oxidising property of H2O2?
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2
2K3[Fe(CN)6] + 2KOH + H2O2 → 2K4[Fe(CN)6] + 2H2O + O2
PbO2 + H2O2 → PbO + H2O + O2
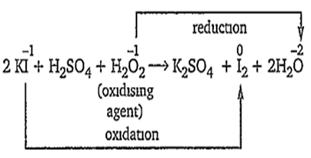
2KI + H2SO4 + H2O2 → K2SO4 + I2 + 2H2O
D.
2KI + H2SO4 + H2O2 → K2SO4 + I2 + 2H2O
The reaction in which H2O2 is reduced while the other reactant is oxidised, represents the oxidsing property of H2O2.
The correct order of reactivity of hydrogen halides with ethyl alcohol is
HF > HCl > HBr > HI
HCl > HBr > HF > HI
HBr > HCl > HI > HF
HI > HBr > HCl > HF
The orange coloured compound formed when H2O2 is added to TiO2 solution acidified with conc. H2SO4 is
Ti2O3
H2Ti2O8
H2TiO3
H2TiO4
How many mL of perhydrol is required to produce sufficient oxygen which can be used to completely convert 2 L of SO2 gas to SO3 gas?
10 mL
5 mL
20 mL
30 mL
Which property among the following is same for both hydrogen and deuterium molecules?
Bond energy
Melting point
Boiling point
Bond length
In which of the following reactions hydrogen is not liberated?
Reaction of fused NaOH with C
Reaction of NaOH with sulphur
Heating the concentrated NaOH with Si
Reaction of zinc with NaOH
Observe the following statements-
i. Heavy water is harmful for the growth of animals.
ii. Haevy water reacts with Al4C3 and forms deyterated acetylene.
iii. BaCl2.2D2O is an example of interstitial deuterate.
The correct statements are-
1 and 3
1 and 2
1, 2 and 3
2 and 3
