 Long Answer Type
Long Answer TypeAnionic Addition Polymerization: Styrene undergoes anionic polymerization easily because C6H5 group in styrene is electron withdrawing. It involves following steps:
(i) Chain initiation step: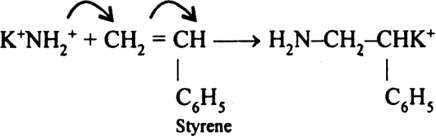
(ii) Chain propagation step: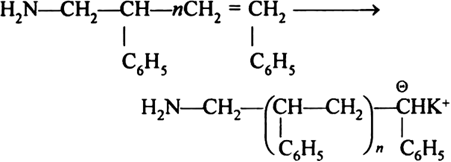
(iii) Chain termination step: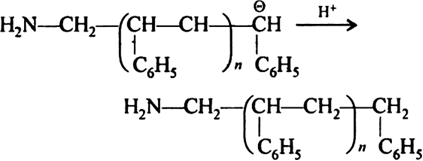
Give the monomers and uses of each of the following addition polymers:
(a) Polyethylene
(b) Polypropylene
(c) Polystyrene.
What are linear polymer and branched chain polymers? How do these differ from own linked polymers?
 Short Answer Type
Short Answer TypeDefine a polymer. Write the monomer used for the preparation of dacron. Mention a use of it.
Write the names and molecular structure of the monomers of the following:
(i) Natural rubber (ii) Neoprene.
Write the names and structures of the monomers of the following polymers:
(i) Polystyrene (ii) Neoprene.
