 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow are low density polyethylene and high density polyethylene manufactured? Why do they differ in their densities?
Low density polyethylene : It is obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atomspheres at a temperature of 350 K to 570 K in the presence of traces of dioxygen or a peroxide initiator. It is chemically inert and tough but flexible and a poor conductor of electricity.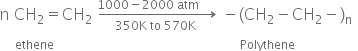
High density polythene : It is formed when addition of polymerisation of ethene takes place in a hydrocarbon solvent in the presence of a catalyst such as triethylaluminium and titanium tetrachloride (Ziegler- Natta catalyst) at temperature of 333K to 343K and under a pressure of 6-7 atm. It is also chemically inert and more tougher and harder.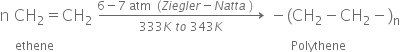
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite the equation for the synthesis of melamine polymer. Mention one important use of this polymer.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhich polymer is used for making plastic crockery? Give its monomer, polymerization and uses.
 Short Answer Type
Short Answer TypeDifferentiate between number average and weight average molecular masses of polymers.
