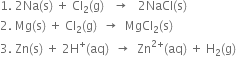Long Answer Type
Long Answer TypeShow that oxidation cannot occur without reduction.
Or
Show that oxidation and reduction go side by side.

 Short Answer Type
Short Answer TypeIdentify the oxidant and the reductant in the following reactions:
In reaction (i) zinc donates electrons to O to give zinc and oxide ions. Therefore, Zn acts as a reductant while oxygen acts as an oxidant.
In reaction (ii) Zn transfers its electrons to H+ and therefore, zinc acts as a reductant and H+ acts as an oxidant.
 Long Answer Type
Long Answer TypeWhat are the changes which take place when a redox reaction is carried in a beaker? Explain with the help of a suitable example.
Or
Explain the redox reaction
occurring in a beaker.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type