Long Answer Type
Long Answer TypeShow that oxidation cannot occur without reduction.
Or
Show that oxidation and reduction go side by side.

 Short Answer Type
Short Answer Type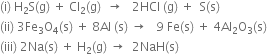
(i) H2S is oxidised because a more electronegative element, chlorine is added to hydrogen or a more electropositive hydrogen has been removed from S and chlorine is reduced because of the addition of hydrogen to it.
(ii) Aluminium is oxidised due to the addition of oxygen to it and ferrous ferric oxide (Fe3O4) is reduced due to the removal of oxygen from it.
(iii) Sodium is oxidised while hydrogen is reduced.
 Long Answer Type
Long Answer TypeWhat are the changes which take place when a redox reaction is carried in a beaker? Explain with the help of a suitable example.
Or
Explain the redox reaction
occurring in a beaker.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type