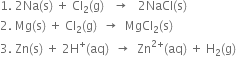Long Answer Type
Long Answer TypeShow that oxidation cannot occur without reduction.
Or
Show that oxidation and reduction go side by side.

 Short Answer Type
Short Answer Type
|
Formula of the compound |
O.N. of metallic element |
Stock notation |
|
HAuCl4 |
3 |
HAu(llI)CI |
|
Tl2O |
1 |
Tl2(I)O |
|
FeO |
2 |
Fe(ll)O |
|
Fe2O3 |
3 |
Fe2(III)O3 |
|
CuI |
1 |
Cu(I)I |
|
CuO |
2 |
Cu(II)O |
|
MnO |
2 |
Mn(II)O |
|
MnO2 |
4 |
Mn(IV)O2 |
 Long Answer Type
Long Answer TypeWhat are the changes which take place when a redox reaction is carried in a beaker? Explain with the help of a suitable example.
Or
Explain the redox reaction
occurring in a beaker.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type