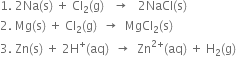Long Answer Type
Long Answer TypeShow that oxidation cannot occur without reduction.
Or
Show that oxidation and reduction go side by side.

 Short Answer Type
Short Answer Type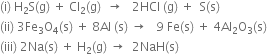
How will you classify the redox reactions?
The redox reactions have been classified into two types:
(i) Direct redox reactions: In these reactions, oxidation and reduction take place in the same vessel. For example,
(a) Displacement of copper from CuSO4 solution when a zinc rod is dipped in it.
(b) Reduction of HgCl2 to Hg2Cl2 by SnCl2.
(ii) Indirect redox reactions: In these reactions, oxidation and reduction take place in different vessels. These reactions form the basis of the electrochemical cells in which chemical energy is converted into electrical energy.
 Long Answer Type
Long Answer TypeWhat are the changes which take place when a redox reaction is carried in a beaker? Explain with the help of a suitable example.
Or
Explain the redox reaction
occurring in a beaker.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type