 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type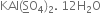
Let the oxidation number of S =x
Oxidation number of K=+1
Oxidation number of Al =+3
Oxidation number of O= (-2)
In this case, we ignore water molecule since it is a neutral molecule.
1+3+2x+[2(-2)4] =0
4+2x+(-16) =0
2x-12=0
x=6
