 Long Answer Type
Long Answer TypeBalance the following equation by oxidation number method: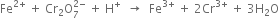
(i) The skeleton equation along with oxidation number of each atom is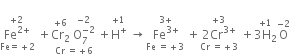
Total O.N. of Cr = +12
Total O.N of Cr = +6
(ii) Noting the change in O.N. of above atoms,
Increase Fe = +2 to +3 to i.e. 1
Decrease Cr = +12 to +6 i.e. 6
(iii) Equalise the increase/decrease in O.N. by multiplying Fe2+ by 6,

(iv) Balance Fe atoms by multiply Fe3+ by 6.

(v) Balance the hydrogen atoms
Net charge on LHS = +12 -2 + 1 = +11
Net charge on RHS = +18 + 6 = +24
So difference = 24 - 11 = 13
Therefore adding thirteen H+ LHS,
(vi) Balance O atoms and four more H2O molecules on RHS, the balanced equation is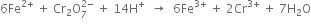
With the net ionic equation for the reaction of potassium dichromate (VI), K2Cr2O7 with sodium sulphite, Na2SO3, in an acid solution to give chromium (III) ion and the sulphate ion.
