 Short Answer Type
Short Answer TypeA 1.00 molar aqueous solution of trichloroacetic acid (CCl3COOH) is heated to its boiling point. The solution has the boiling point of 100.180C. Determine the van’t Hoff factor for trichloroacetic acid. (Kb for water = 0.512 kg mol-1)
Define the following terms:
(i) Mole fraction
(ii) Isotonic solutions
(iii) Van’t Hoff factor
(iv) Ideal solution
(i) Mole fraction: The mole fraction of a component in a mixture is defined as the ratio of the number of moles of the component to the total number of moles of all the components in the mixture. Mathematically, it is represented as:
Mole fraction is denoted by ‘x’.
(ii) Isotonic solution: It is a type of solution that has the same salt concentration as its surrounding environment and thus the substances around it neither lose nor gain water by osmosis.
(iii) Van’t Hoff factor: It is defined as the ratio of the experimental value of colligative property to the calculated value of the colligative property and is used to find out the extent of dissociation or association.
Mathematically, it is represented as: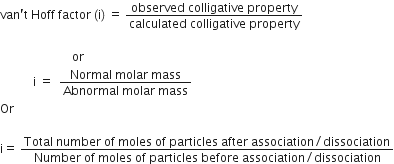
For association, i < 1
For dissociation, i > 1
No association or dissociation, i = 1
Examples: One formula unit of NaCl will create two particles in solution, a Na+ ion and a Cl- ion.
One formula unit of CaCl2 will create three particles in solution, a Ca+ ion and two Cl- ions.
(iv) Ideal Solutions: Ideal Solutions are those which obey Raoult's Law at all concentrations and Temperatures. Some examples of ideal solution liquid pairs are benzene and toluene, n-heptane and n-hexane, ethyl bromide and ethyl iodide, chlorobenzene and bromobenzene etc.
Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (Kf for water = 1.86 K kg mol-1)
 Long Answer Type
Long Answer Type(a) Differentiate between molarity and molality for a solution. How does a change in temperature influence their values?
(b) Calculate the freezing point of an aqueous solution containing 10.50 g of MgBr2in 200 g of water. (Molar mass of MgBr2 = 184 g) (Kf for water = 1.86 K kg mol-1)
(a) Define the terms osmosis and osmotic pressure. Is the osmotic pressure of a solution a colligative property? Explain.
(b) Calculate the boiling point of a solution prepared by adding 15.00 g of NaCl to 250.0 g of water. (Kb for water = 0.512 K kg mol-1), (Molar mass of NaCl = 58.44 g)
 Short Answer Type
Short Answer TypeWhy does a solution containing non-volatile solute have higher boiling point than the pure solvent? Why is elevation of boiling point a colligative property?
Calculate the freezing point of the solution when 31 g of ethylene glycol (C2H6O2) is dissolved in 500 g of water. (Kf for water = 1.86 K kg mol–1)
 Long Answer Type
Long Answer Type(a) State Raoult’s law for a solution containing volatile components.
How does Raoult’s law become a special case of Henry’s law?
(b) 1·00 g of a non-electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0·40 K. Find the molar mass of the solute. (Kf for benzene = 5·12 K kg mol-1)
(a) Define the following terms:
(i) Ideal solution
(ii) Azeotrope
(iii) Osmotic pressure
(b) A solution of glucose (C6H12O6) in water is labeled as 10% by weight. What would be the molality of the solution?
(Molar mass of glucose = 180 g mol-1)
a) Define the following terms:
(i) Mole fraction
(ii) Ideal solution
(b) 15.0 g of an unknown molecular material is dissolved in 450 g of water. The resulting solution freezes at - 0.34°C. What is the molar mass of the material? (Kf for water = 1.86 K kg mol-1)
