 Short Answer Type
Short Answer TypeIf law of constant composition is true, what weights of calcium, carbon and oxygen are present in 1.5 g fo calcium carbonate? Given that a sample of calcium carbonate from another sample contains the following percentage composition:
Ca = 40.0%; C = 12.0%; O= 48.0%
 Long Answer Type
Long Answer TypeThe following data were obtained when dinitrogen and dioxygen react together to form different compounds:
Mass of dinitrogen Mass of dioxygen
(i) 14g 16g
(ii) 14g 32g
(iii) 28g 32g
(iv) 28g 80g
which law of chemical combination is obeyed by the above experimental data? Give its statement?
Carbon and oxygen are known to form two compounds. The carbon content in one of these is 42·9% while in the other it is 27·3%. Show that this data is in agreement with the law of multiple proportions.
Define the law of reciprocal proportion. Explain with suitable examples.
The law of reciprocal proportion (Richtev 1792) states that if two elements A and B combine separately with another element C, then the ratio of respective weights of A and B which combine with a fixed weight of C is the same or simple multiple of the weights in which A and B combine with each other. For example, sodium and chlorine combine with hydrogen to form compounds NaH and HCl respectively.
In NaH:
23 g sodium combines with 1 g hydrogen.
In HCl:
35·5 g chloride combines with 1 g hydrogen.
The ratio of masses of sodium and chlorine which combine with a fixed mass of hydrogen (1 g) is 23 : 35·5
Now sodium and chlorine also combine to form NaCl.
In NaCl:
23g sodium combines with 35·5 chlorine in the ratio 23 : 35·5
This is the same ratio which combines with one part of hydrogen in NaH and HCl.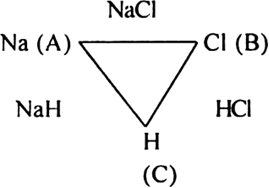
Show that the law of reciprocal proportions is proved by the following results:
1·4g of the element A is known to combine with 1·6g of element B, while 0·5g of another element C combines with 3·5g of the element A and 2·857g of the element C combines with 22·857g of element B.
 Short Answer Type
Short Answer TypeTwo volumes of hydrogen combine with one volume of oxygen to produce two volumes of steam. Show that this illustrates Gay Lussac’s law.
 Long Answer Type
Long Answer Type