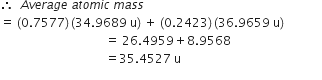Long Answer Type
Long Answer TypeHow Dalton's atomic theory was modified on the basis of modern reseraches? or Give various postulates of modern atomic theory.
Define Avogadro’s law. Taking a suitable example, prove that it is not in contradiction with Dalton’s atomic theory.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeCalculate the atomic mass(average) of chlorine using the following data:
| %Natural Abundance | Molar mass | |
| 35Cl | 75·77 | 34·9689 |
| 37Cl | 24·23 | 36·9659 |