 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat is the empirical formula of the compounds having molecular formula as:
(i) C6H6
(ii) C6H12
(iii) H2O2
(iv) H2O
(v) Na2CO3
(vi) B2H6
(vii) N2O6
 Long Answer Type
Long Answer TypeSilicon (Si = 28) forms a compound with chlorine (Cl = 35·5) in which 5·6g of silicon is combined with 21·3g of chlorine. Calculate the empirical formula of the compound.
2.38 g of uranium was heated strongly in a current of air. The resulting oxide weighed 2806 g. Determine the empirical formula of the oxide. (At. mass of U = 238, O = 16).
Four grams of copper chloride on analysis was found to contain 1·890 g of copper (Cu) and 2·110 g of chlorine (Cl). What is the empirical formula of copper chloride?
A compound on analysis, is found to have the following composition:
(i) Sodium = 14·31%, (ii) Sulphur = 9·97
(iii) Oxygen = 69·50% (iv) Hydrogen = 6·22%.
Calculate the molecular formula of the compound assuming that whole of hydrogen in the compound is present as water of crystallisation. Molecular weight of the compound is 322.

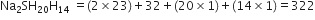

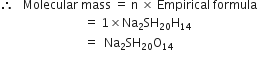
A sample of hydrazine consists of 87·42% N and 12·58% H by mass. The molecular mass of hydrazine is 32·0 g mol–1. Calculate its molecular formula.
