 Multiple Choice Questions
Multiple Choice Questionsx grams of calcium carbonate was completely burnt in air. The weight of the solid residue formed is 28 g. What is the value of x (in grams)?
44
200
150
50
The concentration of oxalic acid is 'x' mol L-1. 40 mL of this solution reacts with 16 mL of 0.05 M acidified KMnO4. What is the pH of 'x' M oxalic acid solution ? (Assume that oxalic acid dissociates completely)
1.3
1.699
1
2
Assertion (A): Equal moles of different substances contain same number of constituent particles.
Reason (R) : Equal weights of different substances contain the same number of constituent particles.
The correct answer is
Both (A) and (R) are true and (R) is the correct explanation of (A)
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false
(A) is false but (R) is true
Match the following:
| List - I | List - II |
| A. 10 gm CaCO3 | (i) 0.224 L CO2 |
| B. 1.06 g Na2CO3 | (ii) 4.48 L CO2 |
| C. 2.4 g C | (iii) 0.448 L CO2 |
| D. 0.56 g CO |
(iv) 2.24 L CO2 (v) 22. 4 L CO2 |
The correct match is
A - iv; B - i; C - ii; D - iii
A - v; B - i; C - ii; D - iii
A - iv; B - i; C - iii; D - ii
A - i; B - iv; C - ii; D - iii
138 g of ethyl alcohol is mixed with 72 g of water. The ratio of mole fraction of alcohol to water is
3 :4
1: 2
1:4
1: 1
1.5 g of CdCl2 was found to contain 0.9 g of Cd. Calculate the atomic weight of Cd.
118
112
106.5
53.25
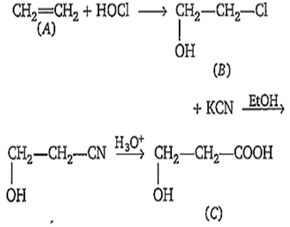
One percent composition of an organic compound A is, carbon : 85.71% and hydrogen 14.29%. Its vapour density is 14. Consider the following reaction sequence-
Identify .
CH3CH(OH)-CO2H
HO-CH2-CH2-CO2H
HO-CH2-CO2H
CH3-CH2-CO2H
B.
HO-CH2-CH2-CO2H
C = 85.71% = = 7.14; = 1
H = 14.29% = = 14.29; = 2
Empirical formula = CH2
and empirical formula weight = 12 + 2 = 14;
Again,
Molecular formula weight = 2 × vapour density
= 2 × 14 = 28
n = = 2
Molecular formula = (CH2)2 = C2H4
At 25°C, the molar conductances at infinite dilution for the strong electrolytes NaOH, NaCl and BaCl2 are 248 × 10-4, 126 × 10-4 and 280 × 10-4 Sm2 mol-1 respectively, (BaOH)2 in Sm2 mol-1 is
52.4 × 10-4
524 × 10-4
402 × 10-4
262 × 10-4
The number of molecules of CO2 liberated by the complete combustion of 0.1 g atom of graphite in air is
3.01 × 1022
6.02 × 1023
6.02 × 1022
3.01 × 1023
19 g of a mixture containing NaHCO3 and Na2CO3 on complete heating liberated 1.12 L of CO2 at STP. The weight of the remaining solid was 15.9 g. What is the weight (in g) of Na2CO3 in the mixture before heating?
8.4
15.9
4.0
10.6
