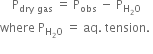Short Answer Type
Short Answer TypeWhat would have been the effect on gas pressure, if the collision between gas molecules were not classic?
How will you account for the fact that gases occupy the whole of the volume available to them?
The tyre of an automobile is inflated to a slightly lesser pressure in summer than in winter. Why?
The size of the weather ballon becomes larger and larger as it ascends to higher altitudes. Explain.
What change is expected if the temperature is kept constant but pressure is reduced to one fourth of its original value?
How do you find the pressure of dry gas collected over water?
By deducting the aqueous tension at that temperature from the observed pressure of the gas.