 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA weather balloon has a volume of 175 L when filled with hydrogen at a pressure of 1.00 bars. Calculate the volume of the balloon when it rises to a height of 2000m, where the atmospheric pressure is 0.80 bar. Assume that the temperature is constant ?
A balloon is filled with hydrogen at room temperature. It will burst if the pressure exceeds 0.2 bar. If at 1 bar pressure, the gas occupies 2.27 L volume, up to what volume can the balloon be expanded?
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30C?
From the given data,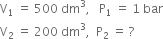
According to Boyle's law,

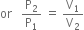
Substituting the values, we have,
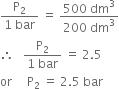
 Long Answer Type
Long Answer TypeA vessel of 120 mL capacity contains a certain amount of gas at 35°C and 1·2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35°C. What would be its pressure ?
 Short Answer Type
Short Answer TypeA gas occupies a volume of 300 mL at 740 mm Hg at 20°C. What additional pressure is required to reduce the gas volume to 250 ml at 20°C?
What pressure must be applied to a given sample of a gas in order to compress it to three-fourth of its original volume ?
