 Long Answer Type
Long Answer TypeHow will you verify Charle's law graphically? What is the practical importance of Charle's law?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA sample of helium gas has a volume of 500 cm3 at 373K. Calculate the temperature at which the volume becomes 260 cm3. Assume that the pressure is kept constant.
On a ship sailing in Pacific ocean where the temperature is 23.4 °C, a balloon is filled with 2L air. What will be the volume of the balloon when the ship reaches Indian ocean, where the temperature is 26.1°C ?
From the given data,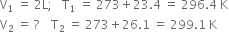
According to Charle's law,

or 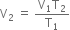
Substituting the values, we have
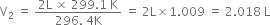
A cylinder contains 450 mL of a gas at 27°C at constant pressure. The cylinder is placed in a refrigerator at –10°C. Calculate the resultant volume of the gas.
What volume of air will be expelled from a vessel containing 380 cm3 at 10°C when it is heated to 30°C at the same pressure ?
 Long Answer Type
Long Answer TypeA student forgot to add the reaction mixture to the round bottomed flask at 27°C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found that the temperature of the flask was 477°C. What fraction of air would have been expelled out?
