 Short Answer Type
Short Answer TypeDensity of a gas is found to be 5.46 g/dm3 atВ 7В°C at 2 bar pressure. What will be its density at STP?В
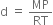
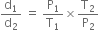 В В В В В В В В В В В ...(1)
В В В В В В В В В В В ...(1)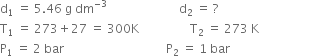
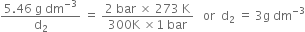
 Long Answer Type
Long Answer TypeIf the density of a gas at the sea level at 0° is 1·29 kg m–3, what is the molar mass? (Assume that pressure is equal to 1 bar).
 Short Answer Type
Short Answer Type34.05 mL of phosphorus vapours weighs 0.0625 g atВ 546В°C and 0В·1 bar pressure. what is the molar mass of phosphorus?
 Long Answer Type
Long Answer Type2.9 g of a gas atВ 95В°C occupied the same volume as 0В·184g of hydrogen at 17В°C at the same pressure. What is the molar mass of the gas?В
 Short Answer Type
Short Answer TypeCalculate the mass of methane in a 9L cylinder at 16 bar and 27°C. (R = 0·08L bar K–1 mol–1).
 Long Answer Type
Long Answer TypeTwo flasks A and B have equal volumes. Flask A contains H2 and is maintained at 300K while the flask B contains an equal mass of CH4 gas and is maintainedВ at 600K. In which flask is the pressure greater? How many times ?
Pressure of 1g of an ideal gas A atВ 27В° C is found to be 2 bar. When 2g of another ideal gas B is introduced in the same flask at same temperature the pressure becomes 3 bar. Find a relationship between their molecular masses.
Pay load is defined as the difference between the mass ofВ displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled With helium at 1В·66 bar at 27В°C. (Density of air = 1В·2 kg m-3В and R = 0В·083 bar dm3В K–1В mo1–1).В
