 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeExplain what is meant by 'critical phenomenon'? What are 'critical constants' of a gas?
Describe briefly the Isotherm of Carbon dioxide.
Or
Briefly describe the Isotherm of Carbon dioxide as studied by Andrews.
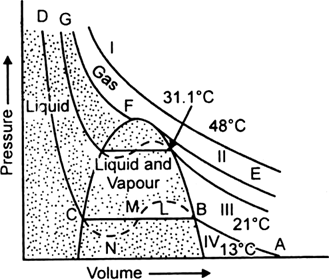
A) Isotherms at a temperature much above the critical temperature (31·1°C): These are very much like the ideal gas isotherms (Isotherm I).
(B) Isotherms at a temperature just above the critical temperature : They have a point of inflexion which is not found in the ideal gas isotherms (Isotherm II).
(C) Isotherms at a temperature just above the critical temperature : These show a straight horizontal part along which the gas gets converted to the liquid state (Isotherms III and IV).
This is the case of discontinuous liquefaction as the properties of the substance in the gaseous and liquid forms are markedly different from each other.
In each of these isotherms, like isotherm (IV) at 13°C, it is clear that with the increase of pressure, the volume of the gas decreases along AB according to Boyle’s law and at B, the liquefaction starts. On decreasing the volume further, there is no change of pressure (along BC) until point C is reached where the whole of the gas has been liquefied.
Curve CD is almost vertical showing that liquid obtained is slightly compressible.
At higher temperature, say 21°C, curve III is a similar isotherm except that horizontal position is shorter. If the temperature is further raised, the horizontal part of the isotherm becomes shorter and shorter until it is reduced to a point F as in curve II at the critical temperature.
Liquefaction of a gas does not occur at all along the isotherm above the critical temperature. It is only along the critical temperature isotherm that a gas undistinguishably continues into the liquid state.
All gases behave similarly except that the length of horizontal portions and peak of the dotted parabola (F) are different depending on the nature of the gas.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeOn the basis of kinetic molecular theory of liquids, how can you explain the following properties of liquids:
(i) Volume (ii) Density
(iii) Compressibility (iv) Diffusion?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeExplain briefly the term "vapour pressure" . What are the factors on which vapour pressure of a liquid depends?
