 Short Answer Type
Short Answer TypeExplain the following:
(i) Drops of liquid assume a spherical shape.
(ii) The level of mercury in a capillary tube is lower than the level outside when a capillary tube is inserted in mercury.
Liquid that wets glass rises in a capillary tube or oil rises in the wick of an oil Jamp. Explain.
 Long Answer Type
Long Answer TypeExplain the following:
(i) The boiling point of water (373 K) is abnormally high when compared to that of H2S (211·2K).
(ii) Liquids like ether and acetone are kept in cool places.
(iii) Tea or coffee is sipped from a saucer when it is quite hot.
Explain briefly the term viscosity. Define coefficient of viscosity. What are its units?
 Short Answer Type
Short Answer TypeWhich one in each of the following pairs is more viscous?
(i) Coconut oil, castor oil
(ii) glyercine, kerosene
(iii) soft drink, aerated water (soda water)?
 Long Answer Type
Long Answer TypeWhat is the effect of temperature on:
(i) density
(ii) surface tension
(iii) viscosity
(iv) the vapour pressure of a liquid?
 Short Answer Type
Short Answer TypeWhat is the effect of pressure on:
(i) volume
(ii) boiling point
(iii) viscosity of a liquid?
 Long Answer Type
Long Answer TypeExplain briefly:
(i) Dispersion or London forces
(ii) Dipole-dipole forces.
(i) Dispersion or London forces: Such types of forces operate between the molecules in all substances. On an average, the electrons or the negative charge of the electron are distributed symmetrically about an atom. For brief periods, there may be some asymmetry in the distribution of electrons around the nucleus resulting into an instantaneous electric dipole which causes an electric field. Due to this field, the electron distribution in the neighbouring atom or molecule is distorted and neighbouring atom or molecule itself acquires a dipole moment. The two dipoles will attract and form the basis of dispersion or London forces. These forces are attractive and interaction energy is directly proportional to 1/r6. Therefore, such forces are important only at short distances (≃ 500 pm). London forces also depend on the polarizability of the molecule.
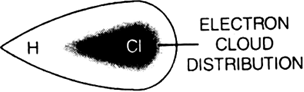
(ii) Dipole-dipole forces: Dipole-dipole forces occur between molecules having a permanent electric dipole. A bond between two dissimilar atoms is polar in nature. For example, in H – Cl molecule, the shared electron pair between hydrogen and chlorine lies closer to the chlorine atom because of its higher electronegativity as compared to a hydrogen atom. Thus, an electric dipole results. This dipole interacts with another neighbouring dipole (hydrogen chloride polar molecule) and produces a net attractive interaction. The interaction energy is directly proportional to 1/r6 where r is the distance between the polar molecules.
 Short Answer Type
Short Answer Type