 Multiple Choice Questions
Multiple Choice QuestionsAs the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following?
1/2
2
313/293
313/293
C.
313/293
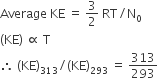
In Vander Waals equation of state of the gas law, the constant ‘b’ is a measure of
intermolecular repulsions
intermolecular collisions per unit volume
Volume occupied by the molecules
Volume occupied by the molecules
An ideal gas expands in volume from 1×10-3 m3 to 1×10-2 m3 at 300 K against a constant pressure of 1×105 Nm-2. The work done is
-900 J
900 J
2780 J
2780 J
For same mass of two different ideal gases of molecular weights M1 and M2, Plots of log V vs log p at a given constant temperature are shown. Identify the correct option.

M1 > M2
M1 = M2
M1 < M2
Can be predicted only if temeperature is known
Which of the following has the dimension if [ML0T-2]?
Coefficient of viscosity
Surface tension
Vapour pressure
Kinetic energy
The boiling points of HF, HCl, HBr and HI follow the order
HF> HCl > HBr > HI
HF> HI> HBr > HCl
HI> HBr > HCl> H
HCl > HF> HBr > HI
At a certain temperature, the value of the slope of the plot of osmotic pressure () against concentration (C in molL-1) of a certain polymer solution is 291R. The temperature at which osmotic pressure is measured, is (R is gas constant)
271°C
18°C
564 K
18 K
For one mole of an ideal gas, the slope of V vs. T curve at constant pressure of 2 atm is X L mol-1 K-1.The value of the ideal universal gas constant 'R' in terms of X is
X L atm mol-1 K-1
x/2 L atm mol-1 K-1
2X L atm mol-1 K-1
2X atm L-1 mol-1 K-1
At a certain temperature the time-required for the complete diffusion of 200 mL of H2 gas is 30 min. The time required for the complete diffusion of 50 mL of O2 gas at the same temperature will be
60min
30min
45min
15min
The RMS velocity of CO gas molecules at 27°C is approximately 1000 m/s. For N2 molecules at 600 K, the RMS velocity is approximately
2000 m/s
1414 m/s
1000 m/s
1500 m/s
