 Short Answer Type
Short Answer TypeThe wavelength of a proton, an electron and an α-particle moving with the same velocity are in the order: electron > proton > α-particle. How do you justify it?
Write the mathematical form of Heisenberg's uncertainty principle.
Heisenberg's mathematical uncertainty principle.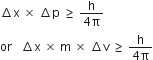
where ∆x is uncertainty in position.
∆p is uncertainty in momentum.
∆v is uncertainty in velocity and h is
Planck’s constant
