 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeA photon of wavelength 4 x 10-7 m strikes on metal surface, the work function of the metal being 2.13 eV. Calculate: (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron (1 eV = 1.6020 10-19J).
(i) Eneryg of one photon ![]()
Substituting the values in eq. (1), we have 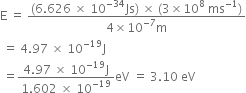
(ii) Kinetic energy of emission 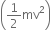
= hv - hv0 = 3.10 - 2.13 = 0.97 eV
(iii) ![]()
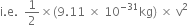
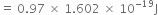
![]()
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe work function for caesium atom is 1.9 eV. Calculate: (a) the threshold wavelength and (b) the threshold frequency of radiation. If the caesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and velocity of the ejected photoelectron.
 Short Answer Type
Short Answer Type