 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeA photon of wavelength 4 x 10-7 m strikes on metal surface, the work function of the metal being 2.13 eV. Calculate: (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron (1 eV = 1.6020 10-19J).
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe work function for caesium atom is 1.9 eV. Calculate: (a) the threshold wavelength and (b) the threshold frequency of radiation. If the caesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and velocity of the ejected photoelectron.
We know
Energy of the incident radiation = Work function + KE of photoelectron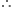 Work function = Energy of the incident
Work function = Energy of the incident
radiation - KE of photoelectron ..(1).
Now energy of the incident radiation (E) = hv
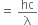 ..(2)
..(2)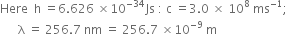
Substituting the values in eq. (2), we have
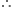 Energy of incident radiation
Energy of incident radiation
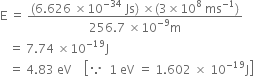
The potential applied gives the kinetic energy to the electron.
Hence, the kinetic energy of the electron = 4.4 eV.
Substituting the values in eq. (1), we have
Work function = 4.83 eV - 0.35 eV
= 4.48 eV
 Short Answer Type
Short Answer Type