 Short Answer Type
Short Answer TypeFind energy of each of the photons which correspond to light of frequency 3 x 1015 Hz.
Here v = 3 x 1015 Hz.

But E = hv
Substituting the values, we have,
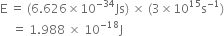
Calcualte the energy of one mole of photons of radiation whose frequency is 5 x 1014Hz.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA 100 watt bulb emits monochromatic light of wavelength 400 nm. Calculate the number of photons emitted per second by the bulb.
Nitrogen laser produces a radiation at a wavelength of 337.1 nm. If the number of photons emitted is 5.6 x 1024, calculate the power of this laser.
 Long Answer Type
Long Answer TypeNeon gas is generally used in the sign boards. If it emits strongly at 616 nm, calculate: (a) the frequency of emission, (b) distance travelled by this radiation in 30 s (c) energy of quantum and (d) number of quanta present if it produces 2 J of energy.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type