 Short Answer Type
Short Answer TypeLifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nano second range. If the radiation source has the duration of 2 ns and the number of photons emitted during the pulse source is 2.5 x 1015, calculate the energy of the source.
Electromagnetic radiation of wavelength 285 nm is just sufficient to ionise the potassium atom. What is the ionization energy of potassium (in kJ mol-1) ?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe energy associated with the first orbit in the hydrogen atom is - 2.18 x 10-18J atom-1. What is the energy associated with the fifth orbit?
(ii) Calculate the radius of Bohr’s fifth orbit for the hydrogen atom.
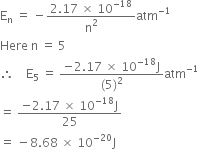
ii) Radius of Bohr’s nth orbit for hydrogen atom is given by,
rn =(0.0529)n2
For
n=5
rs =0.0529nm x 5 x5
rs =1.3225nm
Calculate the energy associated with first orbit of He+. What is the radius of this orbit?
 Long Answer Type
Long Answer TypeHow much energy is required to ionise a H atom if the electron occupies n = 5 orbit? Compare your answer with the ionization enthalpy of H atom (energy required to remove the electron from n = 1 orbit).
 Short Answer Type
Short Answer Type