 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA golf ball has a mass of 40g and a speed of 45m/s. If the speed can be measured within accuracy of 2%, calculate the uncertainty in the position.
 Long Answer Type
Long Answer TypeIf the position of the electron is measured within an accuracy of ![]() calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is
calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is![]() is there any problem in defining this value?
is there any problem in defining this value?
According to Heisenberg’s uncertainty principle.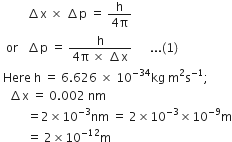
Substituting the values in equation (1), we have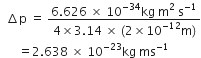
Actual momentum(given) = 
It can not be defined as the actual magnitude of the momentum is smaller than the uncertainty.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type