 Short Answer Type
Short Answer TypeUsing s, p, d notations describe the orbital with the following quantum numbers :
(a) n = 1, l = 0,
(b) n = 3, l = 1,
(c) n = 4, l = 2,
(d) n = 4, l = 3.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type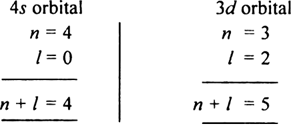
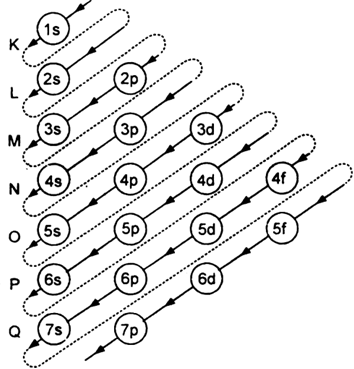
 Short Answer Type
Short Answer TypeThe bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electron experiences the lowest effective nuclear charge?
