 Short Answer Type
Short Answer TypeAmong the following pairs of orbitals which orbital will experience the large effective nuclear charge? (i) 2s and 3s (ii) 4d and 4f (iii) 3d and 3p.
The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus ?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat is Hund's rule of Maximum Multiplicity?
For example, each of the three p-orbitals of the p-subshell, each of the five d-orbitals of d-subshell and each of the seven f-orbitals of the f-subshell gets one electron of parallel spin before any other of them receives the second electron of opposite spin. According to Hund’s rule, the electronic configuration of nitrogen (7N) is
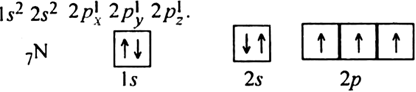
When there is a group of empty orbitals with equal energies (2px, 2py, 2pz), why are electrons first alloted singly to different orbitals ?
 Long Answer Type
Long Answer TypeWrite the electronic configuration of elements from atomic number 11 to atomic number 20. Also name the elements.
Using the Aufbau principle, write the electronic configuration for the ground state of the following atoms:
(i) Boron (Z = 5)
(ii) Neon (Z = 10)
(iii) Al (Z = 13)
(iv) Chlorine (Z = 17)
(v) Calcium (Z = 20).
 Short Answer Type
Short Answer TypeGive the electronic configuration of following ions:
(i) H- (ii) F- (iii) Mg2+ (iv) S2-
