 Short Answer Type
Short Answer Type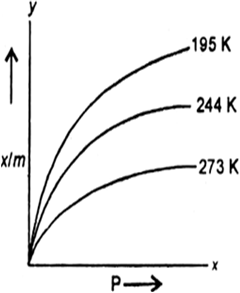
(i) temperature increases at constant pressure.
(ii) pressure increases at constant temperature.
Answer:
The catalytic activity is localised on the surface of the catalyst. The mechanism involves five steps:
(i) Diffusion of reactants to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
(iv) Desorption of reaction products from the catalyst surface, and thereby, making the surface available again for more reaction to occur.
(v) Diffusion of reaction products away from the catalyst’s surface. The surface of the catalyst unlike the inner part of the bulk, has free valencies which provide the seat for chemical forces of attraction. When a gas comes in contact with such a surface, its molecules are held up there due to loose chemical combination. If different molecules are adsorbed side by side, they may react with each other resulting in the formation of new molecules. Thus, formed molecules may evaporate leaving the surface for the fresh reactant molecules.
 Long Answer Type
Long Answer Type