Short Answer Type
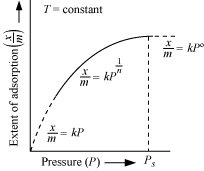
Short Answer TypeThe variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
Freundlich gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation:
x/m = k.p1/n (n > 1)
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n are constants which depend on the nature of the adsorbent and the gas at a particular
temperature.