 Long Answer Type
Long Answer TypeFor M2+/M and M3+/M2+ systems the E° values for some metals are as follows:
Cr2+/Cr – 0.9 V Cr3+/Cr2+ – 0.4 V
Mn2+/Mn – 1.2 V Mn3+/Mn2+ + 1.5 V
Fe2+/Fe – 0.4 V Fe3+/Fe2+ + 0.8 V
Use this data to comment upon
(a) The stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ and
(b) In case with which iron can be oxidised as compared to the similar process for either chromium or manganese metal
Predict which of the following will be coloured in aqueous solution? Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reason for each.
 Short Answer Type
Short Answer Type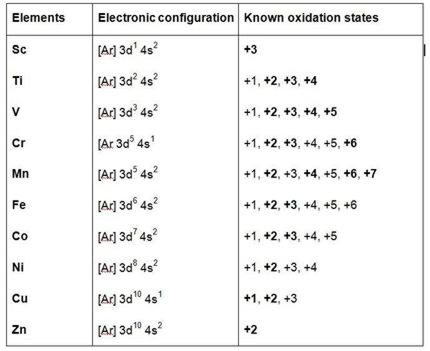
In the beginning of 3d transition series, Sc2+ is virtually not known or in other words it is not stable in comparison to Sc3+, Ti2+, V2+, Cr2+ are known but less stable in comparison to their most common oxidation state of +3.
In the middle Mn2+, Fe2+, Co2+ are known and quite common. In fact Mn2+ and Mn7+ are most stable in Mn. Fe2+ is less stable in comparison to Fe3+ but is due to fact that Fe3+tends to loose one electron to aquire d5 structure which has extra stability. Co2+ is less stable as compared to Co3+. Ni2+ is most common and most stable among its +2, +3, +4 states. Cu2+ is more stable and is most common species as compared to Cu1+. At end Zn forms only Zn2+ which is highly stable as it has 3d10 structure.
Compare the chemistry of actinides with that of the lanthanoids with special reference to:
(i) electronic configuration, (ii) atomic and ionic sizes, (iii) oxidation state (iv) chemical reactivity.
 Long Answer Type
Long Answer TypeHow would you account for the following:
(a) Of the d4 species, Cr2+ is strongly reducing while manganese(III) is strongly oxidising.
(b) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidized.
(c) The d1 configuration is very unstable in ions.
 Short Answer Type
Short Answer Type