 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeUse Hund's rule to derive the electronic configuration of Ce3+ ion, and calculate its magnetic moment on the basis of 'spin only' formula.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeExplain:
The lowest oxidation state of manganese is basic while the highest is acidic.
Explain:
Mn(II) shows maximum paramagnetic character amongst the divalent ions of the first transition series.
 Long Answer Type
Long Answer Type(i) Electronic configuration: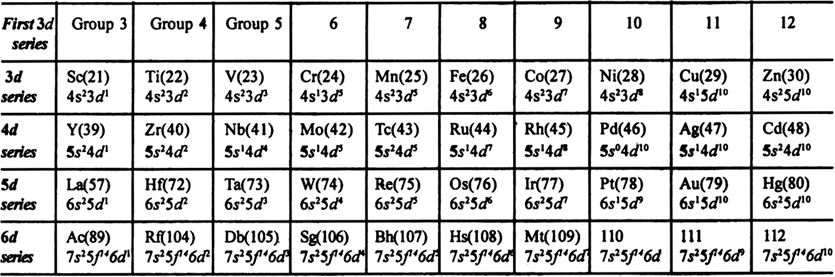
(ii) Oxidation states:
|
Sc Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
|
+2 (+2) +3 +3 +4 |
+2 +3 +4 +5 |
+2 +3 (+4) (+5) +6 |
+2 (+3) +4 (+5) (+6) +7 |
(+1) +2 (+4) (+5) (+6) |
(+1) +2 (+3) (+4) |
+ 1 +2 (+3) (+4) |
+ 1 +2 (+3) |
(+1) +2 |
(iii) Ionization enthalpies
In each of the three transition series, the first ionisation enthalpy increases from left to right. However, there are some exceptions. The first ionisation enthalpies of the third transition series are higher than those of the first and second transition series. This occurs due to the poor shielding effect of 4felectrons in the third transition series.
Certain elements in the second transition series have higher first ionisation enthalpies than elements corresponding to the same vertical column in the first transition series. There are also elements in the 2ndtransition series whose first ionisation enthalpies are lower than those of the elements corresponding to the same vertical column in the 1sttransition series.
|
Element |
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
IE, (kJ mol–1) |
631 |
656 |
650 |
652 |
717 |
762 |
758 |
736 |
745 |
906 |
(iv) Atomic Sizes
Atomic size generally decreases from left to right across a period. Now, among the three transition series, atomic sizes of the elements in the second transition series are greater than those of the elements corresponding to the same vertical column in the first transition series. However, the atomic sizes of the elements in the third transition series are virtually the same as those of the corresponding members in the second transition series. This is due to lanthanoid contraction.
|
Element |
Sc |
Tc |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
Atomic sizes (pm) |
144 |
132 |
122 |
117 |
117 |
117 |
116 |
115 |
117 |
125 |
