 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following does not give oxygen on heating?
KClO3
Zn(ClO3)2
K2Cr2O7
K2Cr2O7
D.
K2Cr2O7
Oxygen-rich compounds like chlorate perchlorate K2Cr2O7 etc. When heated gives oxygen but ammonium dichromate gives nitrogen gas when heated. 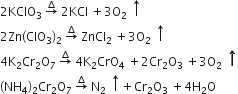
Which of the following lanthanoid ions is diamagnetic?
(At. number; Ce =58, Sm =62, Eu =63, Yb = 70)
Ce2+
Sm2+
Eu2+
Eu2+
KMnO4 can be prepared from K2MnO4 as per reaction
The reaction can go to completion by removing OH- ions by adding
HCl
KOH
CO2
CO2
Which one of the following does not correctly represent the correct order of the property indicated aginst it?
Ti < V<Cr< Mn: increasing number of oxidation states
Ti< V<Cr3+<Mn3+ : increasing magnetic moment
Ti < V < Cr < Mn : Increasing melting points
Ti < V < Cr < Mn : Increasing melting points
Four successive members of the first series of the transition metals are listed below. For which one of them, the standard potential value has a positive sign?
has a positive sign?
Co (Z=27)
Ni (Z=28)
Cu (Z=29)
Cu (Z=29)
The catalytic activity of transition metals and their compounds is ascribed mainly to
their magnetic behaviour
their unfilled d- orbitals
their ability to adopt variable oxidation states
their ability to adopt variable oxidation states
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
(At. no. Cr = 24, Mn = 25, Fe = 26, Co = 27)
Fe > Mn > Co> Cr
Co > Mn > Fe > Cr
Cr > Mn > Co > Fe
Cr > Mn > Co > Fe
Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of
CrO42-
Cr2(SO3)3
CrSO4
CrSO4
Gadolinium belongs to 4f series. Its atomic number is 64. which of the following is the correct electronic configuration of gadolinium?
[Xe]4f8 6d2
[Xe]4f95s1
[Xe]4f7 5d16s2
[Xe]4f7 5d16s2
