 Long Answer Type
Long Answer TypeAluminium trifluoride is insoluble in anhydrous HF but dissolves on the addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reasons.
Boron exhibits anomalous behaviour in the company of other members of group 13. Explain.
 Short Answer Type
Short Answer TypeIn some of the reactions, thallium resembles aluminium whereas in others it resembles with group 1 metals. Support this statement by giving some evidences.
What are borones(boron hybrides)?
(i) Boron does not directly combine with hydrogen but a number of interesting hydrides are known. Boron hydrides such as B2H6 (diborane), B4H10 (tetraborane), B5H9 (pentaborane), B6H10 (hexaborane), B10H14 (decaborane) are collectively called boranes.
(ii) Preparation of diborane:
(a) By reducing boron trifluoride with lithium hybrid. 
(b) By reducing boron trichloride with hydrogen or lithium aluminium hydride.
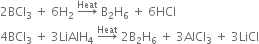
Uses of diborane: It is used:
(i) as fuel for supersonic rockets.
(ii) as a reducing agent in organic reactions.
(iii) for preparing LiBH4, NaBH4 etc.
